Most brain functions require communication between neurons that is mediated by signaling molecules. G protein-coupled receptors (GPCRs) are signaling molecules which are important for most cellular processes. GPCRs are currently the targets for approximately 35% of all drugs in the pharmaceutical industry. Adhesion-class GPCRs are important signaling molecules in the brain and potential treatment of various brain diseases may be possible by better understanding how these receptors function. A major aim of our laboratory is to reveal the mechanism by which adhesion GPCRs function using a combination of structural, biochemical and functional approaches.

Adhesion GPCRs
- With 33 members in humans, adhesion GPCRs (aGPCRs) are the second-largest family in the GPCR superfamily.
- Similar to conventional GPCRs, they have seven-pass transmembrane regions.
- Unlike conventional GPCRs, aGPCRs are characterized by their large, multidomain extracellular regions that mediate cell adhesion.
- All aGPCRs have an extracellular GAIN domain and most are autoproteolysed within the GAIN domain.
- Physiological roles for aGPCRs include brain development, synapse formation/maintenance, myelination of neurons, central nervous system angiogenesis, and cell polarity in neural development as well as other developmental functions.
- aGPCRs are liked to nervous system conditions such as Tourette Syndrome, Autism Spectrum Disorder, Schizophrenia, neurodevelopmental conditions such as ADHD, Usher Syndrome, Joubert Syndrome, spina bifida, polymicrogyria, scoliosis, and cancers such as glioblastoma.
What is the GAIN domain?
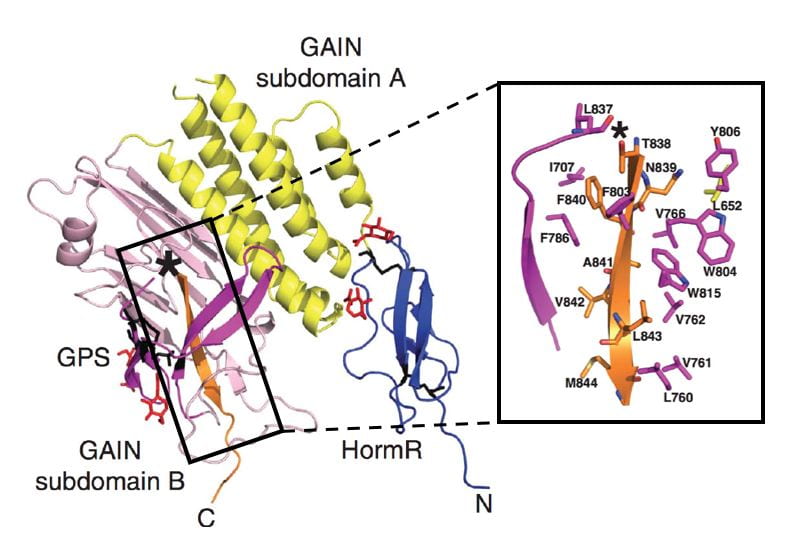
The GPCR Autoproteolysis-INducing (GAIN) domain is an evolutionarily conserved, novel fold that was discovered by our laboratory.
- It is the only extracellular domain shared by all human adhesion GPCRs (except GPR123).
- It is required and sufficient for autoproteolysis.
- It is in close association with the transmembrane region and may regulate receptor signaling.
Research Interests
There are numerous areas to be investigated in order to get a comprehensive understanding of aGPCR function. Our studies focus on different structural elements of the aGPCRs:
- Revealing the role of the extracellular region in receptor function and understanding its mechanism of action to regulate receptor signaling.
- Characterizing other extracellular regions of aGPCRs.
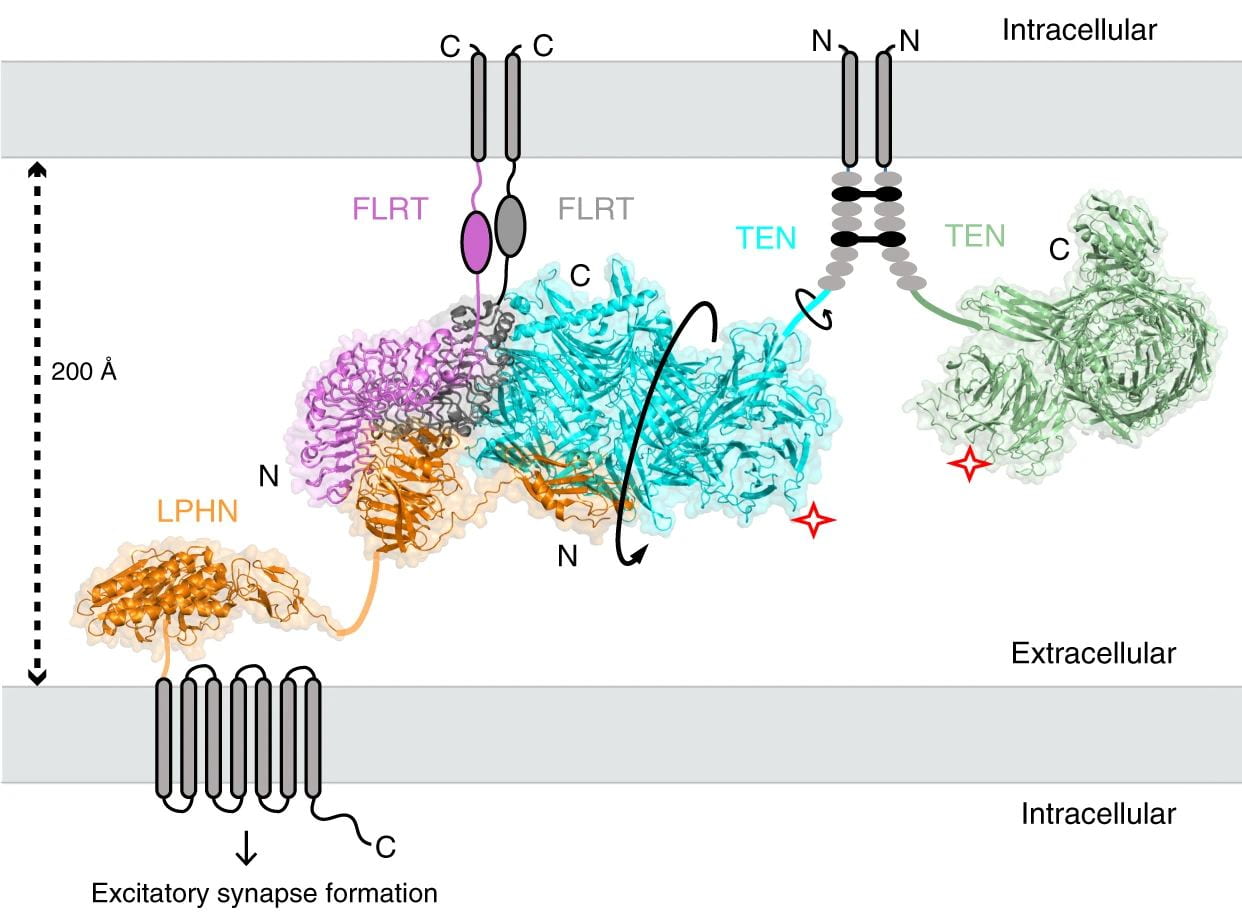
- Discovering aGPCR binding partners and understanding the effect of ligand binding on receptor signaling.
- Investigating the interplay between the extracellular domains and the transmembrane region to better understand how the receptor function can be regulated.
We use a combination of x-ray crystallography, cryogenic electron microscopy, eukaryotic expression of recombinant proteins, biophysical methods, biochemical assays, signaling assays and functional methods to address these questions.
